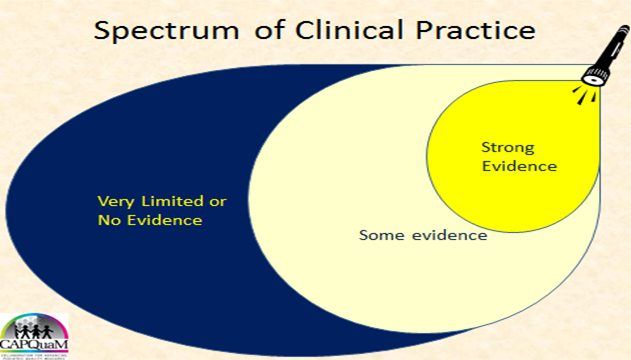
A. Illustrating the limitations of evidence on its own.

Collaboration for the Advancement of Pediatric Quality Measures
The Mount Sinai Collaboration for Advancing Pediatric Quality Measures (CAPQuaM) is one of seven Centers of Excellence funded through the Pediatric Quality Measures Program (PQMP) by the Agency for Health Care Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS). CAPQuaM collaborates with a variety of leading institutions and organizations in this highly-engaged pediatric measure development project. CAPQuaM employs a stakeholder-involved multi-phase approach, in which experts across a variety of consumer domains provide insight and expertise to develop and enhance measures of quality pediatric care.
The underlying philosophy of CAPQuaM is that the state of the evidence is rarely perfect - indeed it typically requires extrapolations and judgments in order to come to a practical understanding of what is high quality care. CAPQuaM seeks to make this process explicit, through a rigorous and systematic methodology that emphasizes the integration of engagement with a wide variety of stakeholders that is informed by the research literature.

A. Illustrating the limitations of evidence on its own.
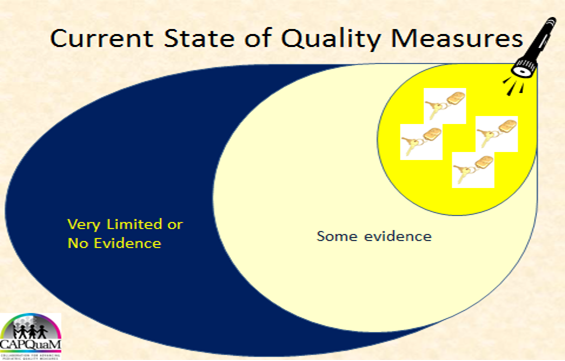
B. Illustrating that measures are typically designed where the light of evidence is brightest.
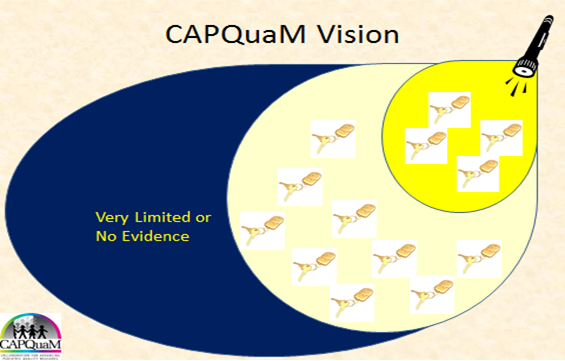
C. Illustrating CAPQuaM's goal of expanding measures into those areas for which there is a meaningful amount of evidence but for which there are still some gaps in how the scientific literature can guide us.
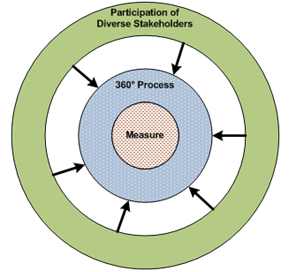
CAPQuaM's 360°Process
CAPQuaM calls its method a 360° approach to reflect its involvement of a wide range of key stakeholders in meaningful ways throughout the process. The resulting measure is at the center of a process transparent to all participants at all stages; the inclusive nature of the development process conveys legitimacy to the measures. The 360° approach seeks to target relevant information and perspective and to have measures emerge from the process. That is, recognizing that practice extends well beyond the research base, we designed this method to allow us to develop reliable and valid state-of-the-science measures, in part by explicitly modeling and accounting for uncertainties in the measure development, especially through the conceptualization and implementation of a Boundary Guideline.
CAPQuaM faculty at Icahn School of Medicine at Mount Sinai
Lawrence Kleinman, MD, MPH, Director and Principal Investigator, CAPQuaM; Vice Chair for Research and Education Department of Population Health Science and Policy, Professor of Population Health Science & Policy and Pediatrics, Icahn School of Medicine at Mount Sinai
Dr. Kleinman is a pioneer and national leader in the measurement and improvement of the quality of health care, particularly health care for children. He also is widely recognized as a research methodologist for both health services and patient-centered outcomes research and as a leading thinker about how to produce generalizable knowledge from community engaged research. He is co-Director of the Mount Sinai CTSA community engagement core and chaired the Context subcommittee of the Outcomes Workgroup of the Community Engagement Key Function Committee for the national CTSA consortium.
He has published several landmark papers on the quality of pediatric care, another on analyzing research data and has developed innovative performance measures for the private sector. An accomplished educator, he directs Mount Sinai's Primary Care Research Fellowship Program (a HRSA funded T32). The Mount Sinai Collaboration for the Advancement of Pediatric Quality Measures (CAPQuaM) is one of seven national Centers of Excellence federally funded by AHRQ, in collaboration with CMS, as part of the federal Pediatric Quality Measures Program created by the Child Health Insurance Reauthorization Act (CHIPRA).
As Vice Chair of the Department of Population Health Science & Policy at the Mount Sinai School of Medicine Dr. Kleinman continues to perform research in support of his vision of an equitable high performing health care system that improves the health of Americans.
Barbara Rabin, MHA, MT(ASCP)SC, BB Assistant Professor, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai
CAPQuaM Staff
| Amy Balbierz, MPH Project Manager |
| Elise Barrow, MPH Project Manager |
| Allisyn Vachon, MPH Clinical Research Coordinator |
| Shannon Weber, BS Research Assistant |
Steering Committee
| Mount Sinai Faculty Leads (for CAPQuaM): |
| Perinatal and High Risk Obstetrics |
| Allisyn Vachon, MPH Clinical Research Coordinator |
| Elizabeth Howell, MD, MPP Associate Professor, Obstetrics, Gynecology and Reproductive Science; Associate Professor Population Health Science and Policy; Associate Professor Psychiatry, Icahn School of Medicine at Mount Sinai |
We propose to establish a collaborative, multidisciplinary center that will develop, investigate, and enhance shared decision making (SDM) and patient-centered care through the use of a boundary approach to clinical discourse between clinicians and families. My research focuses on patient centered outcomes in maternal and child health with special attention to the needs and experiences of low-income women of color. I have conducted research on racial/ethnic disparities in postpartum depression, infant mortality, patient satisfaction, postpartum women's health, and the contribution of racial segregation to prematurity outcomes. I ran two NIH funded randomized controlled trials to reduce racial/ethnic disparities in maternal depression and improve quality of maternal care. I am currently a co-investigator for the Mount Sinai Collaboration for Advancing Pediatric Quality Measures (CAPQuaM) and am helping develop patient centered quality measures in neonatal and obstetrical care. I am Vice-Chair for the Committee on Health Care for Underserved Women of American College of Obstetricians and Associate Director of the Center for Health Equity and Community Engaged Research at Mount Sinai. I am also on the Joint Commission's technical advisory panel for elective deliveries. My background as both an obstetrician and health services researcher, my expertise in quality of care and racial/ethnic disparities in maternal and child health, and my experience conducting qualitative and quantitative research make me well positioned to participate in this proposal.
| Asthma and Mental Health Follow-Up |
| Eyal Shemesh, MD Chief of the Division of Behavioral and Developmental Health, Department of Pediatrics, Mount Sinai Medical Center; Associate Professor of Pediatrics, Behavioral Pediatrics, Mount Sinai School of Medicine; Associate Professor of Psychiatry, Icahn School of Medicine at Mount Sinai |
As a triple boarded psychiatrist, pediatrician and a child psychiatrist, the issue of providing care in pediatric settings that promotes adherence to the established plan has been a focus of my research career. I offer expertise in measurement of behavioral health constructs, including the use of both quantitative and qualitative methods to elicit perspectives from patients and their caregivers, as well as specific expertise in developing and testing constructs related to SDM. Shared decision making represents a powerful tool to align patients and clinicians in clinical management. The boundary approach holds great promise to make complex topics accessible in a joint decision aid.
| Medication Reconciliation and Medication Reconciliation in Mental Health |
| Rebecca Anderson, MPH Director of Strategic Projects, Office of Excellence in Patient Care, Mount Sinai Medical Center |
I have a strong background in healthcare administration and management, with specific training and expertise in particular key areas for the CAPQuaM project. During my tenure at Mount Sinai, I have been involved in the design and implementation of quality metrics in both academic and non-academic endeavors. I managed a research study which measured the appropriateness of tympanostomy tube placement in children in several hospitals in the New York City area, as well as studies which looked at time to treatment for intestinal obstruction, ectopic pregnancy and appendicitis. As a result of these previous experiences, I am acutely aware of the need for process and outcome measures that are evidence-based and specific. My hospital-based experience has taught me the value of collaboration, data-driven decision making and patient-centered care. My administrative and management experience has me understand the importance of project planning, including adherence to timelines and budgets. I have been a co-author on several publications from prior work.
| Mount Sinai Faculty: |
| Andrew Ting, MD Assistant Professor Pediatrics, Pulmonary and Critical Care, Icahn School of Medicine at Mount Sinai |
| Harold Kaplan, MD Senior Faculty, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai |
| Ian Holtzman, MD Chief of Newborn Medicine, Vice Chair for Clinical Affairs, Department of Pediatrics, Professor of Pediatrics, Newborn Medicine and Professor of Obstetrics, Gynecology and Reproductive Science, Ethics Committee Chair, Icahn School of Medicine at Mount Sinai |
| Adam Vella, MD, FAAP Associate Professor of Emergency Medicine, Associate Professor of Pediatrics, Associate Professor of Medical Education, Icahn School of Medicine at Mount Sinai |
| Lynne Richardson, MD Professor of Emergency Medicine, Professor of Population Health Science and Policy, Vice Chair for Academic, Research and Community Programs, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai |
| Carolyn Rosen, MD Assistant Professor Pediatrics, Associate Program Director Pediatric Residency Program, Icahn School of Medicine at Mount Sinai |
| Sandeep Sharma, MD Postdoctoral Fellow |
| External Stakeholders: |
| American Academy of Family Physicians: |
| Wilson Pace, MD, Director, American Academy of Family Physicians National Research Network |
| American Academy of Pediatrics: |
| Lynn Olson, PhD Director of the Department of Research, American Academy of Pediatrics |
| Keri Thiessen, MEd, Senior Health Policy Analyst Health Care Quality Improvement |
| Steven Kairys, MD, Director Quality Improvement and Innovations Network |
| American Academy of Pediatrics |
| American Institutes for Research: |
| Marla Clayman, PhD, MPH |
| Shoshanna Sofaer, Dr.PH |
| Child and Adolescent Health Measurement Initiative, Department of Population, family, and Reproductive Health, Bloomberg School of Public Health, Johns Hopkins University: |
| Christina Bethell, PhD, MBA, MPH, Director |
| Institute for Patient and Family-Centered Care: |
| Marie Abraham, MA Senior Policy and Program Specialist |
| Institute for Patient and Family-Centered Care |
| National Committee for Quality Assurance: |
| Mary Barton, MD, MPP Vice President Performance Measurement |
| National Committee for Quality Assurance |
| National Institute for Children's Health Quality: |
| Charles J. Homer, MD, MPH Chief Executive Officer and President |
| National Institute for Children's Health Quality |
| New York State Department of Health: |
| Foster Gesten, MD Medical Director Office of Health Insurance Programs |
| Joe Anarella, MPH Deputy Division Director, Quality Measure and Improvement |
Senior Advisory Board
| Arthur Aufses, MD Professor of Surgery and Professor of Health Policy Mount Sinai School of Medicine |
| Scott Breidbart, MD Chief Medical Officer, Empire BlueCross BlueShield |
| Wendy Brennan, MS Executive Director, National Alliance on Mental Illness of New York City |
| John R. Clarke, MD, FACS Professor of Surgery, Drexel University; Clinical Director, Patient Safety and Quality, ECRI |
| Sean Currigan, PhD, MPH Senior Director, Strategic Health Care Initiatives at the American Congress of Obstetricians and Gynecologists |
| Martin Hatlie, JD CEO Project Patient Care |
| Beverly Johnson President and Chief Executive Officer of the Institute for Patient and Family Centered Care |
| Marilyn Kacica, MD, MPH Medical Director, Division of Family Health, New York State Department of Health |
| Barbara Kupferman Vice President of Quality Management HealthPlus/Amerigroup, United Healthcare |
| Marc Lashley, MD, FAAP Pediatrician, Allied Pediatrics of New York |
| Kathleen Nelson, MD Clinical Professor of Pediatrics, Associate Chair for Faculty Development, Children's Hospital Los Angeles, Keck School of Medicine, University of Southern California |
| Doris Peter, PhD Director Health Ratings Center; Principal Investigator, Best Buy Drugs at Consumer Reports |
| Laural Pickering, MPH President and CEO Northeast Business Group on Health |
| Harold A. Pincus, MD Professor of Psychiatry, Columbia University; Director of Quality and Outcomes Research, New York-Presbyterian Hospital; Associate Director, Irving Institute for Clinical/Translational Research |
| Eric Rose, MD Professor Population Health Science and Policy, Professor Cardiothoracic Surgery, Professor of Medicine Cardiology, Professor of Surgery, Mount Sinai School of Medicine |
| John Santa, MD, MPH Medical Director of Consumer Reports Health Ratings Center |
| Lisa Simpson, MB, BCh, MPH, FAAP President and CEO of Academy Health |
| Robert St. Peter, MD President and CEO, Kansas Health Institute |
| Ruth Stein, MD Professor of Pediatrics, Albert Einstein College of Medicine |
| Jeffrey Terry, MBA Managing Principal GE Healthcare |
| Paul Wise, MD, MPH Richard E. Behrman Professor of Child Health and Society, Senior Fellow Freeman Spogli Institute for International Studies, Professor of Pediatrics – Neonatal and Developmental Medicine, Professor, by courtesy, Health Research & Policy, Stanford University |
CAPQuaM Measures
High Risk Obstetric Services
Measure Name
High risk deliveries at facilities with 24/7 in-house physician capable of safely managing labor and delivery, and performing a cesarean section, including an emergent cesarean section
Measure Description
Percent of high risk deliveries that are delivered at a facility with 24/7 in-house physician coverage that is dedicated to obstetrics, and includes a physician capable of safely managing labor and delivery, and performing a cesarean section, including an emergent cesarean section.
Detailed Measure Specifications
Measure Name
High risk deliveries at facilities with 24/7 in-house physician coverage dedicated to the obstetrical service by an anesthesiologist who is qualified to provide obstetrical anesthesia
Measure Description
Percent of high risk deliveries that are delivered at a facility with 24/7 in-house physician coverage that is dedicated to the obstetrical service by an anesthesiologist who is qualified to provide obstetrical anesthesia.
Detailed Measure Specifications
Measure Name
High risk deliveries at facilities with 24/7 in-house blood banking/transfusion services available
Measure Description
Percent of high risk deliveries that are delivered at a facility with 24/7 in-house blood banking/transfusion services available.
Detailed Measure Specifications
Measure Name
High risk deliveries at facilities with level 3 or higher NICU services on campus
Measure Description
Percent of high risk deliveries that are delivered at a facility with level 3 or higher NICU services on campus.
Detailed Measure Specifications
Measure Name
Availability of outpatient maternal fetal medicine and specialty care for women with high risk pregnancies
Measure Description
The extent to which high risk pregnant women who have outpatient visits with a maternal fetal medicine specialist or specialist during their pregnancy.
Detailed Measure Specifications
Measure Name
Availability of multidisciplinary outpatient care for women with high risk pregnancies
Measure Description
The percentage of high risk pregnant women seen by at least three specified types of clinicians during their pregnancy.
Detailed Measure Specifications
Measure Name
Assessing the availability of the preconception component of High Risk Obstetrical Services by Estimating the Use of Teratogenic Medications Before and During Pregnancy
Measure Description
The frequency with which teratogenic medications are dispensed to women before and during pregnancy.
Detailed Measure Specifications
Perinatal
Measure Name
Timely temperatures for all low birthweight neonates
Measure Description
Describes the percent of live-born neonates less than 2500 grams that have a temperature documented within the Golden Hour from birth to 60 minutes of age.
Detailed Measure Specifications
Measure Name
Timely temperatures upon arrival in Level 2 or higher nurseries for Low BirthWeight Neonates
Measure Description
Describes the percent of live-born neonates less than 2500 grams that have a temperature documented within 15 minutes after their arrival to a Level 2 or higher nursery.
Detailed Measure Specifications
Measure Name
Distribution of temperatures for low birthweight neonates admitted to Level 2 or higher nurseries in the first 24 hours of life
Measure Description
Describes the distribution of temperatures of live-born neonates less than 2500 grams that arrive to a Level 2 or higher nursery.
Detailed Measure Specifications
Measure Name
Thermal condition of low birth weight neonates admitted to Level 2 or higher nurseries in the first 24 hours of life
Measure Description
Stratifies live-born neonates less than 2500 grams that arrive to a Level 2 or higher nursery on the basis of admission temperature. Strata are cold (≤34.5), very cool (34.51-35.50), cool (35.51-36.50), euthermic (36.51-37.50) and overly warm (> 37.5).
Detailed Measure Specifications
Asthma
Measure Name
Rate of Emergency Department Visit Use for Children Managed for Persistent Asthma
Measure Description
This measure describes the incidence rate of emergency department visits for children ages 2-21 who are being managed for persistent asthma.
Detailed Measure Specifications
Measure Name
Distribution of Emergency Department Visit Use for Children Managed for Persistent Asthma
Measure Description
This measure describes four aspects of the population of children who have persistent for asthma: the number who have emergency department visits; the distribution of visits; and the number of children with persistent asthma and the amount of time each contributes to the person-time denominator of the incidence rate measure in the same set.
Detailed Measure Specifications
Measure Name
Primary Care Connection Prior to Emergency Department Visits For Children with Identifiable Asthma
Measure Description
This measure characterizes care that precedes Emergency Department visits for children ages 2-21 who can be identified as having asthma, using the specified definitions. We sought to identify children with ongoing asthma who should be able to be identified by their health care providers and/or health care plans as having asthma. The operational definition of an identifiable asthmatic is a child who has utilized health care services that suggest the health care system has enough information to conclude that the child has an asthma diagnosis that requires ongoing care.
Detailed Measure Specifications
Measure Name
Primary Care Connection After Emergency Department Visits for Asthma
Measure Description
This measure seeks to capture important aspects of follow up after ED visits for asthma, including prompt follow up with primary care clinicians and prescription fills for controller medications.
Detailed Measure Specifications
Measure Name
Appropriateness of Emergency Department Visits for Children and Adolescents with Identifiable Asthma
Measure Description
This measure estimates the proportion of emergency department visits for asthma that meet criteria for the ED being the appropriate level of care among all ED visits for asthma in children and adolescents with identifiable asthma.
Detailed Measure Specifications
Other PQMP Sites (taken from AHRQ Website):
| AHRQ-CMS CHIPRA Quality Measurement, Evaluation, Testing, Review, and Implementation Consortium (Q-METRIC). |
| Principal Investigator: Gary Freed, MD, MPH, Percy and Mary Murphy Professor of Pediatrics, School of Medicine; Director, Division of General Pediatrics; Director, Children's Health Evaluation and Research (CHEAR) Unit, University of Michigan, Ann Arbor. |
| Description: The AHRQ-CMS CHIPRA Q-METRIC is led by the Department of Pediatrics, University of Michigan, Ann Arbor. Partners include Altarum Institute; HealthCore, Inc; National Association of Children's Hospitals and Related Institutions; National Initiative for Children's Healthcare Quality; Vanderbilt University; Michigan Medicaid; and the American Board of Pediatrics. The AHRQ-CMS Q-METRIC project will work with the National Alliance on Mental Illness (NAMI) and Family Voices to ensure involvement of families and patients. |
| AHRQ-CMS CHIPRA Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN). |
| Principal Investigator: Rita Mangione-Smith, MD, MPH, Professor, Department of Pediatrics, University of Washington and Investigator, Center for Child Health, Behavior and Development, Seattle Children's Research Institute, Seattle, WA. |
| Description: : The AHRQ-CMS CHIPRA COE4CCN is led by Seattle Children's Research Institute. Partners include RAND Corporation, Cincinnati Children's Hospital Medical Center, Children's Hospital Los Angeles, Kaiser Permanente, the Washington State Department of Social and Health Services, the Community Pediatric Foundation of Washington, the Minnesota State Department of Human Services, the Minnesota Academy of Pediatrics Foundation, and Minnesota Family Voices. The AHRQ-CMS CHIPRA COE4CCN project will work with the Washington and Minnesota Medicaid programs, health care providers from the Washington and Minnesota State chapters of the American Academy of Pediatrics, and family and patient representatives from Family Voices of Minnesota to ensure involvement of multiple stakeholders in all phases of quality measure development. |
| AHRQ-CMS CHIPRA Pediatric Measurement Center of Excellence (PMCoE). |
| Principal Investigator: Ramesh Sachdeva, MS, PhD, JD, Professor of Pediatrics, Executive Vice President and Chief Operation Officer, National Outcomes Center, Children's Hospital and Health System, Milwaukee, WI, and Chief Quality Officer, Children's Hospital and Health System of Wisconsin. |
| Description: The AHRQ-CMS CHIPRA PMCoE is led by the Medical College of Wisconsin/National Outcomes Center; partners include the American Medical Association (AMA)-Convened Physician Consortium for Performance Improvement (PCPI), American Board of Medical Specialties, American Board of Pediatrics, Northwestern University Feinberg School of Medicine, American Academy of Pediatrics (AAP), Thomson-Reuters, TMIT Consulting, LLC, and the Chicago Pediatric Quality and Safety Consortium. The AHRQ-CMS CHIPRA PMCoE project will work with the Illinois Department of Health and Family Services and with the AMA-PCPI Consumer-Purchaser Advisory Panel, the AAP collaboration with Family Voices, and the National Partnership for Women and Families to ensure involvement of families. |
| AHRQ-CMS CHIPRA National Collaborative for Innovation in Quality Measurement (NCINQ). |
| Principal Investigator: Sarah Hudson Scholle, DrPH, Vice President, National Committee for Quality Assurance. |
| Description: AHRQ-CMS CHIPRA NCINQ is led by the National Committee for Quality Assurance; partners include Nationwide Children's Hospital; New York State Department of Health; New York State Office of Mental Health; American Academy of Pediatrics; Rutgers Center for Health Services Research on Pharmacotherapy, Chronic Disease Management, and Outcomes; Child and Adolescent Health Measurement Initiative; and the National Alliance to Advance Adolescent Health. The AHRQ-CMS CHIPRA NCINQ project will work with clinical settings serving disadvantaged children, including Children's Health Fund, the New York City Health Department's Primary Care Information Project, and Hudson River HealthCare, as well as eight State Medicaid/CHIP programs (New York, Illinois, Florida, Minnesota, Oregon, Alaska, West Virginia, and Colorado) and CHIPRA quality demonstration grantees in Illinois and Florida. The AHRQ-CMS CHIPRA NCINQ will have representatives from the National Partnership for Women and Families, Family and Youth Voices, Mental Health America, the National Alliance on Mental Illness, and the National Federation of Families for Children's Mental Health to ensure involvement of families and patients. and patients. |
| AHRQ-CMS CHIPRA Children's Hospital Boston Center of Excellence for Pediatric Quality Measurement (CEPQM). |
| Principal Investigator: Mark A. Schuster, MD, PhD, William Berenberg Professor of Pediatrics, Harvard Medical School, and Chief of the Division of General Pediatrics and Vice Chair for Health Policy in the Department of Medicine at Children's Hospital Boston. |
| Description: The lead organization for this CoE is Children's Hospital Boston (CHB). Partners include Harvard Medical School, Harvard Pilgrim Health Care, Harvard School of Public Health, Massachusetts General Hospital, RAND, Mathematica Policy Research, and the University of Massachusetts Boston. CEPQM is also working with Atrius Health, Blue Cross Blue Shield of Massachusetts, Cambridge Health Alliance, Children's Hospital Association, Massachusetts Health Quality Partners, Massachusetts League of Community Health Centers, Mass Health (Medicaid/CHIP), Pediatric Physicians' Organization at Children's, and the Pediatric Research in Inpatient Settings (PRIS) Network. CEPQM's efforts are also informed by several external advisory groups including a Scientific Advisory Board with representatives from CHB, the larger Harvard community, and organizations such as the National Initiative for Children's Healthcare Quality; and a National Stakeholder Panel, with representatives from a diverse group of national organizations representing payers, providers, patients/families, and health services researchers, including Family Voices, the American Academy of Pediatrics, and the American Hospital Association. CEPQM also benefits from a partnership with the Massachusetts Child Health Quality Coalition, comprising representatives from academic, State, and community institutions, as well as parents and patient advocates, such as the New England Alliance for Children's Health, Boston Public Health Commission, Boston Public Schools, and the Parent/Professional Advocacy League to ensure involvement of families, patients, and community members. |
| AHRQ-CMS CHIPRA Center of Excellence at the Children's Hospital of Philadelphia (CHOP) /University of Pennsylvania (UPenn). |
| Principal Investigator: Jeffrey H. Silber, MD, PhD, Director, Center for Outcomes Research, The Children's Hospital of Philadelphia, Professor of Pediatrics and Anesthesiology and Critical Care at the University of Pennsylvania School of Medicine, and Professor of Health Care Management at the Wharton School. |
| Co-Principal Investigator: Christopher B. Forrest, MD, PhD, Professor of Pediatrics at the University of Pennsylvania School of Medicine. |
| Description: The lead organization for this AHRQ-CMS CHIPRA CoE is the Children's Hospital of Philadelphia. Partners include Medicaid/CHIP leadership from several States including Pennsylvania, Massachusetts, and New Jersey; as well as the State University of New York at Stony Brook (SUNY), Temple University, the Nemours Children's Hospital, and the University of Colorado (participating institutions of the Pediatric EHR Data Sharing Network (PEDSNet), and the Center for Health Care Strategies. The Center of Excellence at CHOP/UPENN will also collaborate with the Public Citizens for Children and Youth (PCCY), which will provide insight from families and children who experience Pennsylvania's public insurance programs. |